Thermal Physics
An enormous advance in the understanding of thermodynamics enabled the great developmments in technology of the industrial revolution in the nineteenth century, and it is hugely important today to allow us to meet our society's energy needs in a sustainable way. Getting a handle on this topic is of great importance for grasping the difficulties and limits of much of modern technology, and even physical theory itself. In this topic, you will find out how gases behave and how this can be related to the kinetic behaviour of their particles, how temperature and heat energy are related, and how heat energy is transferred in temperature changes and changes of state.
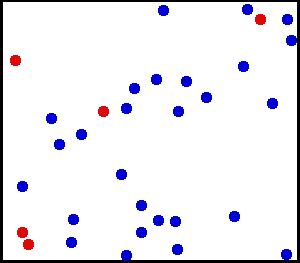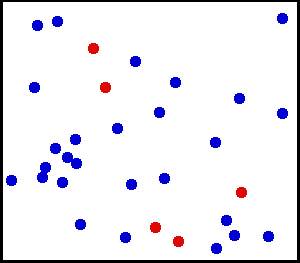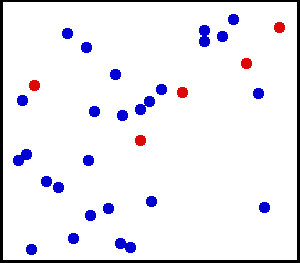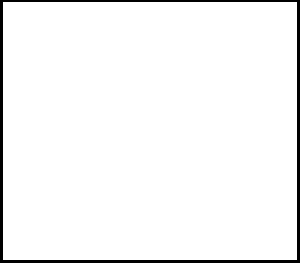
The thermal motion of helium atoms in the gaseous phase. The size of the atoms relative to their spacing is shown to scale under 1950 atmospheres of pressure. These room-temperature atoms have been slowed down two trillion fold. Five atoms are colored red to facilitate following their motions. [CREDIT: Greg L at the English language Wikipedia]
Lessons
- 12 Mar: Phases of matter, pV=nRT
- 13 Mar: The experimental gas laws
- 20 Mar: Charles' law experiment (ISA preparation)
- 25 Mar: Pressure–temperature investigation (ISA preparation)
- 26 Mar: ISA theory question preparation
- Easter vacation
- 17 Apr: Specific heat capacity and specific latent heat
- 22 Apr: Molecular Kinetic Theory I
ISA Preparation
Pressure–volume relationship for gases
You are going to investigate how the volume of a fixed mass of gas changes when the pressure is changed.

